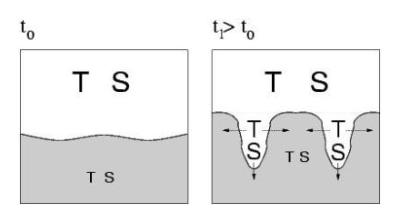
Mechanism of "finger" formation (e.g. at outflow of the Mediterranean over the Atlantic):
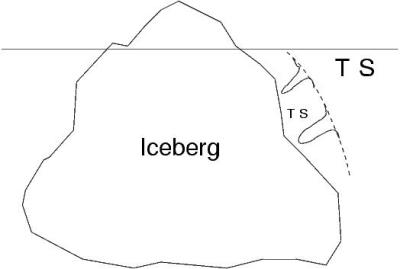
Another configuration:
Structures due to double-diffusive convection appear in: the outflow of
the Mediterranean over the Atlantic, low-and mid-latitude oceans due to
surface heating (they are considered by oceanographers as the leading cause
of salt transport), the manufacture of alloys or of semiconductor crystals
out of the melt (leading to unwanted bands), icebergs towed to warmer coastal
regions (structures there hinder the desidered fresh water supply), seawage
assumed to be disposed safely at the bottom of the sea (even if heavier
than water, the formation of salt fingers may push dangerous pollutants
upwards), polymers transported in the extracellular matrix of living tissues,
the magma below the earth's crust, and the interior of stars ("helium fingers").
Example (see diagramm below):
A salty, hot layer (large S and T) is above a colder and fresher layer.
The upper layer is alltogether lighter, and thus gravitationally stable.
However, since heath diffuses faster than the salt S, a fluctuation causing
a small protrusion downwards rapidly cooles down, so that S can sink: a
"finger" grows.
Mechanism of "finger" formation (e.g. at outflow of the Mediterranean over the Atlantic):

Another configuration:

We have figured out a simple way of generating, stabilizing and visualizing such finger growth in the laboratory. A drop consisting of a mixture of glycerine, an alcohol ethoxylate (a surfactant) and water is injected at the bottom of a petri dish filled with water. The situation is that shown above, but turned upside down. T corresponds to glycerine and S to the surfactant.
The gravity-induced expansion of the drop induces a flow in the sorrounding water, as indicated by the closed arrows below. The fingers emerging from the drop follow the flow at the bottom of the dish, and are thus confined quasi two-dimensionally, facilitating visualization. In the figures below, the fingers appear as thin, needle-shaped structures. The surfactant S forms large aggregates (micelles), which diffuse extremely slowly,so that S stays in the fingers until these structures reach the border of the dish. This is in contrast with lateral instabilities of fingers reported so far in the literature. The stability and the 2D-confinement of the structures allow analyses of video recordings that permit quantitative comparisons with our calculations using partial differential equations.
|
|
|
|
Detailed view of figure above:
|
Water flow induced by the expanding drop:
|
Publications
M. Markus and K. Kötter: "Doppelt-diffusive Konvektion". Der mathematische und naturwissenschaftliche Unterricht 58, 24-28 (2005).
M. Markus, "Double-diffusive convection: a simple demonstration", J. Chem. Ed. 81, 526-529 (2004).
K. Koetter, M. Schmick and M. Markus: "Transport Out of a Gravitationally Stable Layer with the Help of a Faster Diffusing Substance: PDE Simulations and Scaling Laws". In: Interface And Transport Dynamics. (Ed. by H. Emmerich, B. Nestler & M. Schreckenberg), 322-328, Springer, Berlin (2003).
K. Koetter and M. Markus, "Power laws for sizes and growth times of double-diffusive convection cells", International Journal of Heat and Mass Transfer 45, 5045-5051 (2002)
K. Koetter and M. Markus, "Scaling Laws for the Wavelength and the Appearance Time of Cells Resulting from Double-Diffusive Convection", Science Asia 28, 49-54 (2002)
K. Koetter and M. Markus, "Double-diffusive fingering instability of a surfactant-glycerine-water drop in water", Europhys. Lett. 55, 807-813 (2001)
M. Markus and K. Koetter, "Of icebergs and coffee creamers", Education in Chemistry, July, 108 (2001)
M. Markus and K. Koetter, "Die Salzfinger von Gibraltar auf dem Labortisch", UNI-Report 30, Univ. Dortmund, 54-55 (2000)